Dangerous Drugs
Baby Powder Lawsuit Information
More than 1,000 women and their families are suing Johnson & Johnson (J&J), claiming that the company knew about the potential risk of developing ovarian cancer from using its baby powder in the genital area yet failed to warn consumers. J&J never issued any warnings about risks associated with genital use of talcum powder. One jury ordered the company to pay $72 million to the family of a woman who died of ovarian cancer after using J&J’s baby powder for years.
Invokana Lawsuit Information
A study done by the Institute for Safe Medication Practices identified 457 serious adverse events with canagliflozin, the active ingredient in Invokana, as the suspect drug. This total was higher than 92% of the drugs they regularly monitor. As a result, Invokana lawsuits are alleging that the makers of the diabetes drug failed to warn patients about potentially serious side effects.
Pradaxa Lawsuit Information
Boehringer Ingelheim agreed to pay $650 million to settle thousands of lawsuits involving its blood thinner Pradaxa. These Pradaxa lawsuits claim that the company failed to properly warn patients and their families that Pradaxa could cause serious and sometimes fatal bleeding events, and that these bleeding events could not be easily reversed.
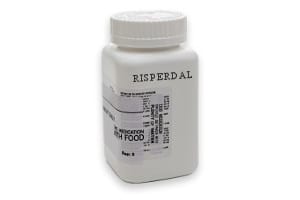
Risperdal Lawsuit Information
Gynecomastia, or enlarged male breasts, has been linked to the use of the anti-psychotic prescription medication Risperdal by young men. Unfortunately, for a large number of these men who’ve taken Risperdal surgery is the only treatment for their gynecomastia. However, the embarrassment these men experience may be worse than any pain and suffering that could come from surgery.
Taxotere Lawsuit Information
Taxotere lawsuits claim that Sanofi-Aventis, the manufacturer, failed to warn doctors and cancer patients that use of the chemotherapy drug could lead to an increased risk of permanent alopecia (loss of hair). Taxotere lawyers are questioning why doctors and patients were never warned of these risks. Given this information, many cancer patients may have chosen a drug which was just as effective but did not carry the risk of this devastating side effect.
Testosterone Therapy Lawsuit Information
The U.S. Food and Drug Administration (FDA) released a safety alert requiring manufacturers of approved testosterone therapy products to change their labeling. The new labels are to include language about a possible increased risk of heart attack and stroke with use of the product. The FDA also cautioned that testosterone therapy products should only be used by men with low testosterone levels caused by certain medical conditions.
Xarelto Lawsuit Information
Xarelto lawsuits are alleging that there is a risk of uncontrollable bleeding associated with the use of Xarelto and the makers of the blood thinner failed to warn of these risks. Xarelto attorneys are also claiming that the makers of Xarelto neglected to divulge that currently there is no antidote for the blood thinner. That means that should an uncontrollable bleed occur, it will be difficult to stop.
Zofran Lawsuit and Birth Defect Information
Zofran use during the first trimester of pregnancy may potentially double the chance of a child being born with birth defects. The anti-nausea medication is being recommended to treat severe morning sickness but it has not been approved by the U.S. Food & Drug Administration (FDA) for such use. GlaxoSmithKline, the maker of Zofran, allegedly never confirmed the drug was safe for use in pregnant women before offering it as a treatment for morning sickness.