Stryker Hip Replacement Lawsuits
Stryker Recalls LFIT V40, Rejuvenate & ABG-II Implant Systems
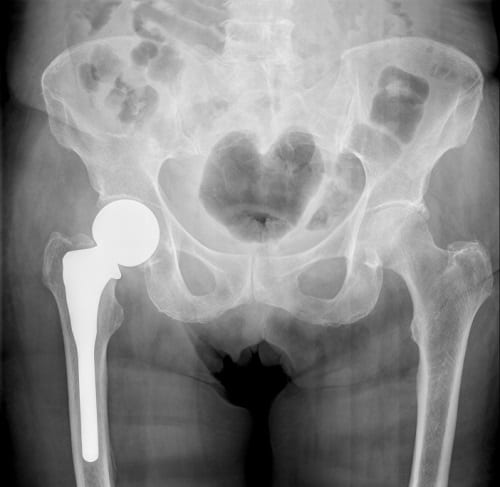 Many manufacturers, including Stryker, issued voluntary recalls for their faulty hip replacement systems. Stryker issued a voluntary recall of its Rejuvenate and ABG-II hip implant systems in June of 2012. At that time the company was concerned about the potential failure of the device as well as an adverse effect on the tissue surrounding the implant. In August 2016, Stryker sent out an urgent medical device recall notification on its LFIT V40 Femoral Head implant. Potential hazards of the LFIT V40 implant include disassociation of femoral head from hip stem and the release of excessive metallic debris into the body. This has been particularly problematic when the LFIT V40 femoral head is combined with an Accolade or Citation stem. These hazards can cause serious complications for patients including the loss of mobility and the need for a revision surgery.
Many manufacturers, including Stryker, issued voluntary recalls for their faulty hip replacement systems. Stryker issued a voluntary recall of its Rejuvenate and ABG-II hip implant systems in June of 2012. At that time the company was concerned about the potential failure of the device as well as an adverse effect on the tissue surrounding the implant. In August 2016, Stryker sent out an urgent medical device recall notification on its LFIT V40 Femoral Head implant. Potential hazards of the LFIT V40 implant include disassociation of femoral head from hip stem and the release of excessive metallic debris into the body. This has been particularly problematic when the LFIT V40 femoral head is combined with an Accolade or Citation stem. These hazards can cause serious complications for patients including the loss of mobility and the need for a revision surgery.
Start Your Free Case Review!
Metal-on-Metal Hip Replacement Side Effects
It has been reported that metal-on-metal hip replacements have the highest failure rate and fail at a rate of over 10%; meaning that many patients will need to endure costly and painful revision surgery.
In addition to device failure, metal-on-metal hip replacements can causes metal ions to enter into bloodstream and create a condition called metallosis. Because these devices are made from a blend of several different metals, they can release metal particles into the blood stream and surrounding tissue when the metal parts rub against each other. A buildup of this type of metal in the body can be toxic, leading to serious complications affecting the nervous system, skin and other organs. Symptoms of metallosis can include:
- General hypersensitivity reaction (skin rash)
- Cardiomyopathy
- Neurological changes including sensory changes (auditory, or visual impairments)
- Psychological status change (including depression or cognitive impairment)
- Renal function impairment
- Thyroid dysfunction (including neck discomfort, fatigue, weight gain or feeling cold)
Other people have experienced pain, swelling, and joint dislocation. If you have a metal-on-metal hip implant and have experienced any of the side effects mentioned above or below, please call our firm:
- Implant Failure
- Revision Surgery
- Elevated levels of cobalt and/or chromium in the blood
- Diagnosis of metallosis
- Loosening of the device
AFFECTED BY A STRYKER HIP?
Call Now! 1-800-710-3121
Stryker Metal-on-Metal Hip Implant Models
- Stryker LFIT V40 (2016 recall)
- Stryker Rejuvenate (2012 recall)
- Stryker ABG-II (2012 recall)
- Stryker Accolade
- Stryker Citation
- Stryker Meridian
- Stryker TMZF Femoral Stem
Are You Eligible for a Stryker Metal Hip Replacement Lawsuit?
The Feeney Law Firm is committed to seeking justice for those affected by metal hip replacements. Fighting to keep people safe from corporate greed and self-interest is our passion. We are here to help make that process as simple as possible for you and your family. Call us now at 1-800-710-3121 for a FREE consultation. Don’t wait; your time to file a metal hip replacement lawsuit may be limited.
